Graphene: a Nobel story
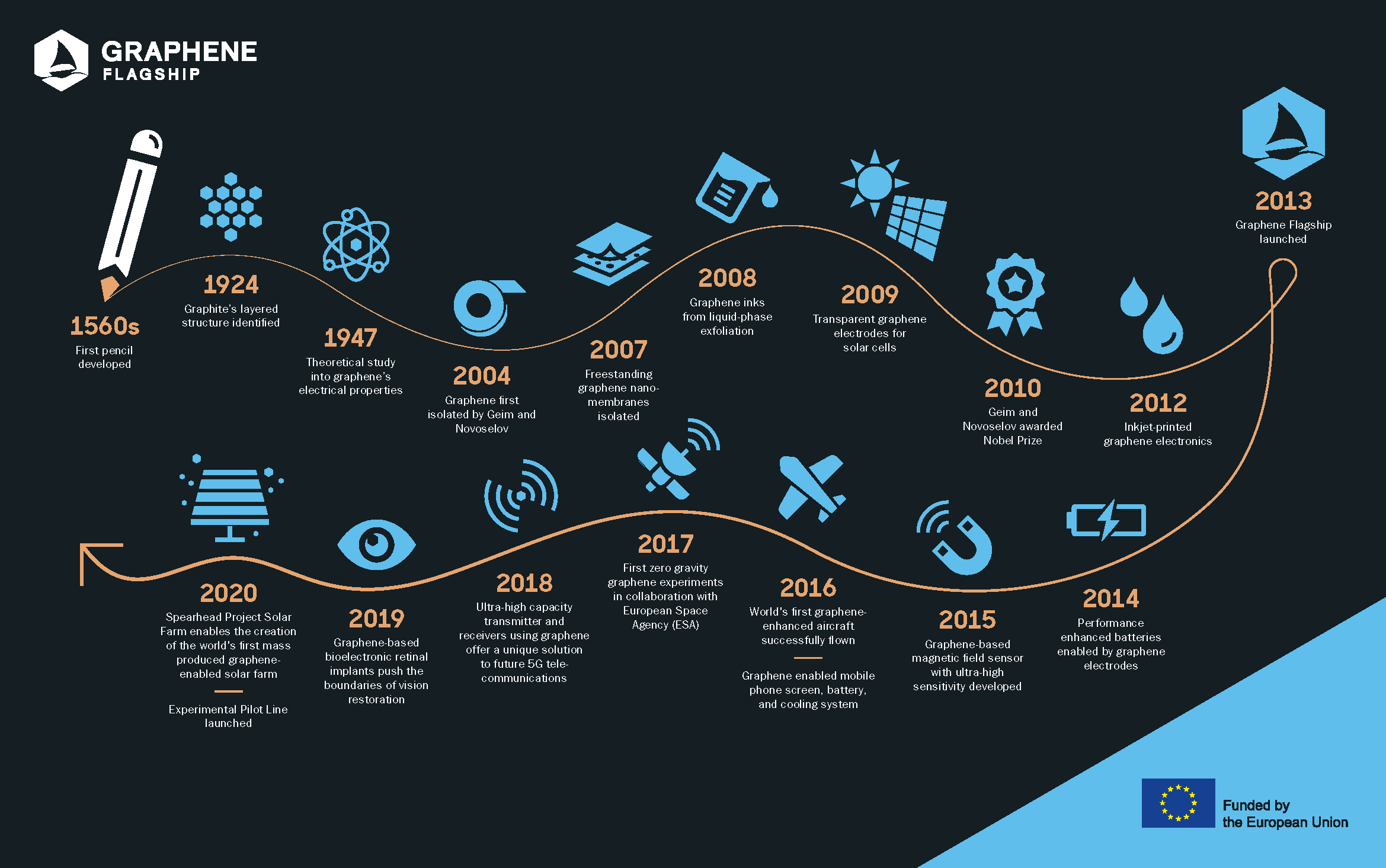
Since 2013, the Graphene Flagship has brought together academic and industrial researchers to push graphene and layered materials to the forefront of European scientific research. But when –and how– did everything begin? Discover the history of graphene. (Picture: Adam Baker, Flickr).